Articles
- Page Path
- HOME > Urogenit Tract Infect > Volume 18(3); 2023 > Article
- Original Article Impact of Microbial Infection on Sperm Parameters of Seminal Bacteria in Asymptomatic Subfertile Males
-
Sae Byuk Chang1
 , Tae Jin Kim2
, Tae Jin Kim2 , Tae Heon Kim1
, Tae Heon Kim1 , Seung-Ryeol Lee1
, Seung-Ryeol Lee1 , Young Kwon Hong1
, Young Kwon Hong1 , Dong Soo Park1
, Dong Soo Park1 , Sun-Mi Cho3
, Sun-Mi Cho3 , Dong Hyeon Lee4
, Dong Hyeon Lee4 , Young Dong Yu1,5,
, Young Dong Yu1,5,
-
Urogenital Tract Infection 2023;18(3):82-92.
DOI: https://doi.org/10.14777/uti.2023.18.3.82
Published online: December 31, 2023
1Department of Urology, Bundang CHA Hospital, CHA Medical University College of Medicine, Seongnam, Korea
2Department of Urology, Il-san CHA Hospital, CHA Medical University College of Medicine, Goyang, Korea
3Department of Laboratory Medicine, Bundang CHA Hospital, CHA Medical University College of Medicine, Seongnam, Korea
4Department of Physiology, CHA Medical University College of Medicine, Seongnam, Korea
5Department of Male Infertility and Urology, Fertility Center of Bundang CHA Hospital, Seongnam, Korea
-
Correspondence to: Young Dong Yu,
 , Department of Urology, Bundang CHA Hospital, CHA Medical University College of Medicine, 59 Yatap-ro, Bundang-gu, Seongnam 13496, Korea, Tel: +82-31-780-5350, Fax: +82-31-780-4959, E-mail: danielyu0714@gmail.com
, Department of Urology, Bundang CHA Hospital, CHA Medical University College of Medicine, 59 Yatap-ro, Bundang-gu, Seongnam 13496, Korea, Tel: +82-31-780-5350, Fax: +82-31-780-4959, E-mail: danielyu0714@gmail.com
Copyright © 2023, Korean Association of Urogenital Tract Infection and Inflammation. All rights reserved.
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 1,294 Views
- 14 Download
Abstract
-
Purpose This study examined the effects of asymptomatic bacteriospermia on the semen quality of subfertile males. The types of bacteria and their antibiotic susceptibility were also analyzed.
-
Materials and Methods Semen was collected and analyzed from 510 subfertile males. One hundred and seventy-nine males showed bacteriospermia, while 331 males did not. The bacterial species, sperm parameters, hormone levels, underlying disease, and lifestyle patterns were compared between the two study groups.
-
Results The bacteriospermic males showed significantly higher rates of leukocytospermia (p=0.001) and deoxyribonucleic acid (DNA) fragmentation than the non-bacteriospermic males. Sperm motility was significantly lower in the bacteriospermic males than in non-bacteriospermic males. The most common
-
Conclusions Bacteriospermia decreased the sperm concentration, motility, normal morphology, and vitality. P. bivia is the most commonly observed bacteria in subfertile males. Appropriate antibiotic therapy of seminal bacteria species had a strong positive impact on improving the semen parameters.
INTRODUCTION
MATERIALS AND METHODS
RESULTS
DISCUSSION
CONCLUSIONS
-
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
FUNDING
No funding to declare.
-
AUTHOR CONTRIBUTIONS
S.B.C. designed the study, analyzed and interpreted the clinical data, and wrote and revised the manuscript. T.J.K., T.H.K., S.R.L., D.S.P., and S.M.C. collected the clinical data and engaged in patient follow‑up. D.H.L. analyzed and interpreted the clinical data. Y.D.Y. designed and supervised the project and revised the manuscript. All the listed authors have participated actively in the study. All authors read and approved the final manuscript.
NOTES
Values are presented as mean±standard deviation (range), number (%), or mean±standard deviation.
LUTS: lower urinary tract symptoms, FSH: follicle-stimulating hormone, LH: luteinizing hormone, DNA: deoxyribonucleic acid, RBCs/HPF: red blood cells/high-power field, PCR: polymerase chain reaction, HSV1: Herpes simplex type-1, HSV2: Herpes simplex type-2, –: not available.
a)p-vlaues <0.05 are printed in bold characters. b)Multi-microbial indicates equal or more than 2 bacterial species present in body fluid. c)1 drink is equivalent to 1 glass of wine or 1 single spirit. d)5-alpha reductase inhibitor was used for the treatment of androgenic alopecia.
Values are presented as number (%).
C. trachomatis: Chlamydia trachomatis, U. parvum: Ureaplasma parvum, U. urealyticum: Ureaplasma urealyticum, M. genitalium: Mycoplasma genitalium, G. vaginalis: Gardnerella vaginalis, A. vaginae: Atopobium vaginae, GBS: Streptococcus agalactiae, P. bivia: Prevotella bivia, E. faecalis: Enterococcus faecalis, M. curtisii: Mobiluncus curtisii, N/A: not applicable, –: not available.
Values are presented as mean±standard deviation or number (%).
U. parvum: Ureaplasma parvum, U. urealyticum: Ureaplasma urealyticum, M. genitalium: Mycoplasma genitalium, G. vaginalis: Gardnerella vaginalis, Tx: treatment, DNA: deoxyribonucleic acid, A. vaginae: Atopobium vaginae, GBS: Streptococcus agalactiae, P. bivia: Prevotella bivia, E. faecalis: Enterococcus faecalis, M. curtisii: Mobiluncus curtisii, C. trachomatis: Chlamydia trachomatis, –: not available.
a)Genitourianry tract bacterial presence indicates bacterial infection confirmed in urine or semen samples. b)Semen parameters are presented in mean±standard deviations. c)Recurrence rate was evaluated at 3 months after the initial antibiotics treatment. d)Post-antibiotics treatment semen analysis was performed on the patient who had no bacterial reinfection at 3 months after the initial antibiotics treatment. e)p-vlaues <0.05 are printed in bold characters.
- 1. Gnoth C, Godehardt E, Frank-Herrmann P, Friol K, Tigges J, Freundl G. Definition and prevalence of subfertility and infer-tility. Hum Reprod 2005;20:1144-7. ArticlePubMed
- 2. Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol 2015;13:37. ArticlePubMedPMCPDF
- 3. Han H, Liu S, Zhou XG, Tian L, Zhang XD. Aetiology of obstruc-tive azoospermia in Chinese infertility patients. Andrologia 2016;48:761-4. ArticlePubMed
- 4. Lackner J, Schatzl G, Horvath S, Kratzik C, Marberger M. Value of counting white blood cells (WBC) in semen samples to predict the presence of bacteria. Eur Urol 2006;49:148-52. discussion 152-3. ArticlePubMed
- 5. Punab M, Loivukene K, Kermes K, Mändar R. The limit of leucocytospermia from the microbiological viewpoint. Andro-logia 2003;35:271-8. ArticlePDF
- 6. Pajovic B, Radojevic N, Vukovic M, Stjepcevic A. Semen analysis before and after antibiotic treatment of asymptomatic Chlamydia- and Ureaplasma-related pyospermia. Andrologia 2013;45:266-71. ArticlePubMed
- 7. Zhang F, Dai J, Chen T. Role of Lactobacillus in female infertility via modulating sperm agglutination and immobilization. Front Cell Infect Microbiol 2021;10:620529. ArticlePubMedPMC
- 8. Fraczek M, Kurpisz M. Mechanisms of the harmful effects of bacterial semen infection on ejaculated human spermatozoa: potential inflammatory markers in semen. Folia Histochem Cytobiol 2015;53:201-17. ArticlePubMed
- 9. Allow AK, Abdulmogny ASS, Maryam B, Alaw BA. Sperm agglutination, sperm shaky head movement and sperm-cervi-cal interaction tests could be enough for diagnosis of immunological infertility? J Gynecol Womens Health 2017;3:555603. Article
- 10. Volz Y, Ebner B, Pfitzinger P, Berg E, Lellig E, Marcon J, et al. Asymptomatic bacteriospermia and infertility-what is the connection? Infection 2022;50:1499-505. ArticlePubMedPMCPDF
- 11. Gimenes F, Souza RP, Bento JC, Teixeira JJ, Maria-Engler SS, Bonini MG, et al. Male infertility: a public health issue caused by sexually transmitted pathogens. Nat Rev Urol 2014;11:672-87. ArticlePubMedPDF
- 12. Fraczek M, Hryhorowicz M, Gill K, Zarzycka M, Gaczarzewicz D, Jedrzejczak P, et al. The effect of bacteriospermia and leukocytospermia on conventional and nonconventional semen parameters in healthy young normozoospermic males. J Reprod Immunol 2016;118:18-27. ArticlePubMed
- 13. Wogatzky J, Wirleitner B, Stecher A, Vanderzwalmen P, Neyer A, Spitzer D, et al. The combination matters--distinct impact of lifestyle factors on sperm quality: a study on semen analysis of 1683 patients according to MSOME criteria. Reprod Biol Endocrinol 2012;10:115. ArticlePubMedPMC
- 14. Pluddemann A. Can drinking more water prevent urinary tract infections? The evidence says yes. BMJ Evid Based Med 2019;24:191-2. ArticlePubMed
- 15. Eini F, Kutenaei MA, Zareei F, Dastjerdi ZS, Shirzeyli MH, Salehi E. Effect of bacterial infection on sperm quality and DNA fragmentation in subfertile men with Leukocytospermia. BMC Mol Cell Biol 2021;22:42. ArticlePubMedPMCPDF
- 16. Domes T, Lo KC, Grober ED, Mullen JB, Mazzulli T, Jarvi K. The incidence and effect of bacteriospermia and elevated seminal leukocytes on semen parameters. Fertil Steril 2012;97:1050-5. ArticlePubMed
- 17. Machado A, Cerca N. Influence of biofilm formation by Gard-ne-rella vaginalis and other anaerobes on bacterial vaginosis. J Infect Dis 2015;212:1856-61. ArticlePubMed
- 18. Farahani L, Tharakan T, Yap T, Ramsay JW, Jayasena CN, Minhas S. The semen microbiome and its impact on sperm function and male fertility: a systematic review and meta-analysis. Andrology 2021;9:115-44. ArticlePubMedPDF
- 19. Bai S, Li Y, Hu MH, Wu L, Shui LJ, Wang XH, et al. Association of sexually transmitted infection with semen quality in men from couples with primary and secondary infertility. Asian J Androl 2022;24:317-22. ArticlePubMed
- 20. Toren P, Margel D, Kulkarni G, Finelli A, Zlotta A, Fleshner N. Effect of dutasteride on clinical progression of benign prostatic hyperplasia in asymptomatic men with enlarged prostate: a post hoc analysis of the REDUCE study. BMJ 2013;346:f2109. ArticlePubMedPMC
- 21. Said MA, Mehta A. The impact of 5α-reductase inhibitor use for male pattern hair loss on men's health. Curr Urol Rep 2018;19:65. ArticlePubMedPDF
- 22. La Vignera S, Condorelli RA, Balercia G, Vicari E, Calogero AE. Does alcohol have any effect on male reproductive function? A review of literature. Asian J Androl 2013;15:221-5. ArticlePubMedPMC
REFERENCES
Figure & Data
REFERENCES
Citations

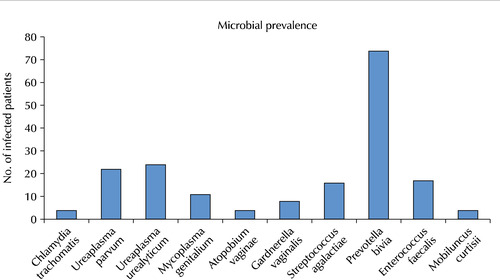
Fig. 1
Basic characteristics of the subfertile males (n=510)
| Total (n=510) | Bacteriospermic subfertile males (n=179) | Non-bacteriospermic subfertile males (n=331) | p-valuea) | |
|---|---|---|---|---|
| Age (y) | 36.6±6.9 (29-44) | 37.3±6.8 (30-44) | 36.1±7.1 (29-43) | 0.510 |
| Underlying disease history | 0.001 | |||
| Hypertension | 10 (2.0) | 3 (1.7) | 7 (2.1) | 0.021 |
| Chronic prostatitis | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Cerebrovascular disease | 2 (0.4) | 0 (0.0) | 2 (0.6) | 0.056 |
| Diabetes mellitus | 24 (4.7) | 19 (10.6) | 5 (1.5) | <0.001 |
| Presence of LUTS | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Previous history of genital surgery | 0.797 | |||
| Spermatocelectomy | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Hydrocelectomy | 9 (1.8) | 3 (1.7) | 6 (1.8) | |
| Orchiopexy | ||||
| Due to testicular torsion | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Due to testicular cryptorchidism | 2 (0.4) | 1 (0.6) | 1 (0.3) | |
| Varicocelectomy | 13 (2.5) | 5 (2.8) | 8 (2.4) | |
| Medical treatment prior to the first visit | ||||
| Clomiphene citrate | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Testosterone replacement therapy | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
| Initial hormone level | ||||
| FSH (mIU/ml) | 5.2±1.5 | 5.1±1.4 | 5.4±0.9 | 0.151 |
| LH (mIU/ml) | 3.8±1.9 | 3.9±1.3 | 3.7±1.7 | 0.337 |
| Testosterone (ng/ml) | 4.8±1.4 | 5.0±1.2 | 4.7±1.1 | 0.242 |
| Prolactin (ng/ml) | 5.6±2.3 | 5.4±2.2 | 5.9±1.9 | 0.508 |
| Semen parameters | ||||
| Volume (ml) | 3.0±2.2 | 2.9±1.7 | 3.6±1.5 | 0.196 |
| Sperm concentration (106/ml) | 46.2±23.6 | 43.3±17.4 | 47.8±20.2 | 0.655 |
| Total motility (%) | 30.0±12.9 | 20.4±10.0 | 36.0±8.6 | <0.001 |
| Progressive motility (%) | 18.1±7.1 | 11.1±5.3 | 22.5±4.7 | <0.001 |
| Leukocytospermia (106/ml) | 0.8±2.1 | 2.8±1.9 | 0.3±0.2 | 0.001 |
| DNA Fragmentation (%) | 32.4±15.2 | 43.2±13.8 | 30.7±11.5 | <0.001 |
| Normal morphology (%) | 2.1±2.3 | 1.5±1.2 | 2.4±1.5 | 0.239 |
| Vitality (%) | 53.0±9.7 | 51.7±6.1 | 55.9±8.4 | 0.575 |
| Microscopic hematuria | <0.001 | |||
| Overall | 125 (24.5) | 115 (64.2) | 10 (3.0) | |
| Grade, RBCs/HPF | ||||
| 0-3 | 100 (19.6) | 93 (51.9) | 7 (2.1) | |
| 4-10 | 20 (3.9) | 17 (9.5) | 3 (0.9) | |
| 11-25 | 5 (1.0) | 5 (2.8) | 0 (0.0) | |
| 26-50 | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Semen pathogen PCR analysis | ||||
| Pathogen spectrum | ||||
| Escherichia coli | 0 (0.0) | |||
| Chlamydia trachomatis | 4 (2.2) | |||
| Neisseria gonorrhoeae | 0 (0.0) | |||
| Ureaplasma parvum | 22 (12.3) | |||
| Ureaplasma urealyticum | 24 (13.4) | |||
| Mycoplasma genitalium | 11 (6.1) | |||
| Mycoplasma hominis | 0 (0.0) | |||
| Trichomonas vaginalis | 0 (0.0) | |||
| Gardnerella vaginalis | 8 (4.5) | |||
| Treponema pallidum | 0 (0.0) | |||
| Candida albicans | 0 (0.0) | |||
| Haemophilus ducreyi | 0 (0.0) | |||
| Atopobium vaginae | 4 (2.2) | |||
| Streptococcus agalactiae | 16 (8.9) | |||
| Prevotella bivia | 74 (41.3) | |||
| Pseudomonas aeruginosa | 0 (0.0) | |||
| Klebsiella pneumoniae | 0 (0.0) | |||
| Enterococcus faecalis | 17 (9.5) | |||
| Staphylococcus aureus | 0 (0.0) | |||
| HSV1 | 0 (0.0) | |||
| HSV2 | 0 (0.0) | |||
| Bacteroides fragilis | 0 (0.0) | |||
| Mobiluncus curtisii | 4 (2.2) | |||
| Mobiluncus mulieris | 0 (0.0) | |||
| Multi-microbialb) | 21 (11.7) | |||
| Pathogen presence in semen, overall | 179 (100.0) | - | ||
| Multi-microbial | 21 (11.7) | |||
| Mono-microbial | 158 (88.3) | |||
| Average alcohol consumptionc) | <0.001 | |||
| 0 drinks/wk | 121 (23.7) | 23 (12.8) | 98 (29.6) | |
| 1-15 drinks/wk | 151 (29.6) | 19 (10.6) | 132 (39.9) | |
| >15 drinks/wk | 238 (46.7) | 137 (76.5) | 101 (30.5) | |
| Smoking history | 0.576 | |||
| Never smoked | 350 (68.6) | 125 (69.8) | 225 (68.0) | |
| Current smoker | 97 (19.0) | 34 (19.0) | 63 (19.0) | |
| Previous smoker (current non-smoker) | 63 (12.4) | 20 (11.2) | 43 (13.0) | |
| 5-alpha reductase inhibitor treatmentd) | 109 (21.4) | 29 (16.2) | 80 (24.2) | 0.039 |
Values are presented as mean±standard deviation (range), number (%), or mean±standard deviation.
LUTS: lower urinary tract symptoms, FSH: follicle-stimulating hormone, LH: luteinizing hormone, DNA: deoxyribonucleic acid, RBCs/HPF: red blood cells/high-power field, PCR: polymerase chain reaction, HSV1: Herpes simplex type-1, HSV2: Herpes simplex type-2, –: not available.
a)p-vlaues <0.05 are printed in bold characters. b)Multi-microbial indicates equal or more than 2 bacterial species present in body fluid. c)1 drink is equivalent to 1 glass of wine or 1 single spirit. d)5-alpha reductase inhibitor was used for the treatment of androgenic alopecia.
Antibiotic sensitivity of pathogens detected in urine or semen samples of subfertile males
| C. trachomatis (n=4) | U. parvum (n=22) | U. urealyticum (n=24) | M. genitalium (n=11) | G. vaginalis (n=8) | A. vaginae (n=4) | S. agalactiae (n=16) | P. bivia (n=74) | E. faecalis (n=17) | M. curtisii (n=4) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Ampicillin | N/A | N/A | N/A | N/A | ||||||
| Sensitive | - | - | - | - | 5 (62.5) | 4 (100.0) | 16 (100.0) | 41 (55.4) | 17 (100.0) | 1 (25.0) |
| Intermediate | - | - | - | - | 1 (12.5) | 0 (0.0) | 0 (0.0) | 10 (13.5) | 0 (0.0) | 0 (0.0) |
| Resistant | - | - | - | - | 2 (25.0) | 0 (0.0) | 0 (0.0) | 23 (31.1) | 0 (0.0) | 3 (75.0) |
| Amoxicillin | N/A | N/A | N/A | N/A | N/A | |||||
| Sensitive | 2 (50.0) | - | - | - | 5 (62.5) | 4 (100.0) | 16 (100.0) | 45 (60.8) | - | - |
| Intermediate | 0 (0.0) | - | - | - | 1 (12.5) | 0 (0.0) | 0 (0.0) | 8 (10.8) | - | - |
| Resistant | 2 (50.0) | - | - | - | 2 (25.0) | 0 (0.0) | 0 (0.0) | 21 (28.4) | - | - |
| Ceftriaxone | N/A | N/A | N/A | |||||||
| Sensitive | 3 (75.0) | - | - | - | 7 (87.5) | 4 (100.0) | 16 (100.0) | 59 (79.7) | 0 (0.0) | 3 (75.0) |
| Intermediate | 0 (0.0) | - | - | - | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (2.7) | 0 (0.0) | 0 (0.0) |
| Resistant | 1 (25.0) | - | - | - | 1 (12.5) | 0 (0.0) | 0 (0.0) | 13 (17.6) | 17 (100.0) | 1 (25.0) |
| Clarithromycin | N/A | N/A | N/A | |||||||
| Sensitive | 4 (100.0) | 10 (45.5) | 12 (50.0) | 5 (45.5) | 6 (75.0) | 4 (100.0) | - | - | - | 4 (100.0) |
| Intermediate | 0 (0.0) | 1 (4.5) | 0 (0.0) | 1 (9.0) | 0 (0.0) | 0 (0.0) | - | - | - | 0 (0.0) |
| Resistant | 0 (0.0) | 11 (50.0) | 12 (50.0) | 5 (45.5) | 2 (25.0) | 0 (0.0) | - | - | - | 0 (0.0) |
| Clindamycin | ||||||||||
| Sensitive | 2 (50.0) | 9 (40.9) | 11 (45.8) | 5 (45.5) | 7 (87.5) | 4 (100.0) | 14 (87.5) | 74 (100.0) | 3 (17.6) | 4 (100.0) |
| Intermediate | 0 (0.0) | 2 (9.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Resistant | 2 (50.0) | 11 (50.0) | 14 (58.3) | 6 (54.5) | 1 (12.5) | 0 (0.0) | 2 (12.5) | 0 (0.0) | 14 (82.4) | 0 (0.0) |
| Amikacin | N/A | N/A | N/A | |||||||
| Sensitive | 4 (100.0) | 22 (100.0) | 24 (100.0) | 11 (100.0) | 8 (100.0) | 4 (100.0) | 16 (100.0) | - | - | - |
| Intermediate | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - | - | - |
| Resistant | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - | - | - |
| Doxycycline | N/A | |||||||||
| Sensitive | 4 (100.0) | 22 (100.0) | 24 (100.0) | 10 (90.9) | 1 (12.5) | 4 (100.0) | 2 (12.5) | - | 5 (29.4) | 2 (50.0) |
| Intermediate | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (12.5) | 0 (0.0) | 0 (0.0) | - | 0 (0.0) | 0 (0.0) |
| Resistant | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (9.1) | 6 (75.0) | 0 (0.0) | 14 (87.5) | - | 12 (70.6) | 2 (50.0) |
| Ciprofloxacin | N/A | N/A | ||||||||
| Sensitive | 3 (75.0) | 10 (45.5) | 10 (41.7) | 4 (36.4) | 3 (37.5) | 3 (75.0) | 15 (93.8) | - | 0 (0.0) | - |
| Intermediate | 0 (0.0) | 0 (0.0) | 2 (8.3) | 3 (27.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - | 0 (0.0) | - |
| Resistant | 1 (25.0) | 12 (54.5) | 12 (50.0) | 4 (36.4) | 5 (62.5) | 1 (25.0) | 1 (6.2) | - | 17 (100.0) | - |
| Metronidazole | N/A | N/A | N/A | N/A | N/A | |||||
| Sensitive | - | - | - | - | 3 (37.5) | 2 (50.0) | 0 (0.0) | 74 (100.0) | - | 0 (0.0) |
| Intermediate | - | - | - | - | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | - | 0 (0.0) |
| Resistant | - | - | - | - | 5 (62.5) | 2 (50.0) | 16 (100.0) | 0 (0.0) | - | 4 (100.0) |
| Piperacillin/tazobactam | N/A | N/A | N/A | N/A | N/A | |||||
| Sensitive | - | - | - | - | 8 (100.0) | - | 16 (100.0) | 74 (100.0) | 17 (100.0) | 4 (100.0) |
| Intermediate | - | - | - | - | 0 (0.0) | - | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Resistant | - | - | - | - | 0 (0.0) | - | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Ertapenem | N/A | N/A | N/A | N/A | N/A | N/A | ||||
| Sensitive | - | - | - | - | - | - | 16 (100.0) | 74 (100.0) | 17 (100.0) | 4 (100.0) |
| Intermediate | - | - | - | - | - | - | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Resistant | - | - | - | - | - | - | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Values are presented as number (%).
C. trachomatis: Chlamydia trachomatis, U. parvum: Ureaplasma parvum, U. urealyticum: Ureaplasma urealyticum, M. genitalium: Mycoplasma genitalium, G. vaginalis: Gardnerella vaginalis, A. vaginae: Atopobium vaginae, GBS: Streptococcus agalactiae, P. bivia: Prevotella bivia, E. faecalis: Enterococcus faecalis, M. curtisii: Mobiluncus curtisii, N/A: not applicable, –: not available.
Pre- and post-antibiotics treatment semen parameters in subfertile males with bacteriospermiaa)
| U. parvum | U. urealyticum | M. genitalium | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||||
| Pre-Tx (n=22) | Post-Tx (n=17)d) | p-valuee) | Pre-T x (n=24) | Post-Tx (n=21) | p-value | Pre-Tx (n=11) | Post-Tx (n=10) | p-value | |||
| Semen parametersb) | |||||||||||
| Volume (ml) | 2.1±1.5 | 2.8±0.8 | 0.144 | 2.2±1.9 | 2.7±2.0 | 0.157 | 2.3±1.3 | 2.9±1.6 | 0.209 | ||
| Sperm concentration (106/ml) | 45.0±12.9 | 48.2±13.4 | 0.528 | 44.8±15.5 | 45.0±14.2 | 0.782 | 43.2±17.0 | 45.0±9.7 | 0.611 | ||
| Total motility (%) | 17.8±3.1 | 41.4±5.7 | <0.001 | 17.5±6.9 | 47.0±10.1 | <0.001 | 18.0±7.7 | 42.1±5.4 | <0.001 | ||
| Progressive motility (%) | 7.9±4.3 | 31.7±5.9 | <0.001 | 7.3±8.2 | 37.1±9.6 | <0.001 | 9.2±5.6 | 32.9±6.2 | <0.001 | ||
| Leukocytospermia (106/ml) | 3.0±1.4 | 0.4±0.4 | <0.001 | 3.1±1.6 | 0.3±0.1 | <0.001 | 3.0±1.2 | 0.5±0.2 | <0.001 | ||
| DNA fragmentation (%) | 44.6±10.5 | 30.2±11.5 | <0.001 | 45.7±8.6 | 31.4±5.3 | <0.001 | 43.1±11.9 | 30.3±7.8 | <0.001 | ||
| Normal morphology (%) | 1.1±0.5 | 1.1±0.9 | 0.560 | 1.1±1.3 | 1.2±1.1 | 0.617 | 1.5±0.8 | 1.5±1.0 | 0.770 | ||
| Vitality (%) | 47.9±6.5 | 50.4±7.3 | 0.284 | 45.2±8.4 | 47.5±10.3 | 0.589 | 46.5±6.1 | 49.0±11.4 | 0.331 | ||
| Recurrence of bacterial infectionc) | - | 5 (31.8) | - | 3 (12.5) | - | 1 (9.1) | |||||
| G. vaginalis | A. vaginae | S. agalactiae | |||||||||
| Pre-Tx (n=8) | Post-Tx (n=8) | p-value | Pre-Tx (n=4) | Post-Tx (n=3) | p-value | Pre-Tx (n=16) | Post-Tx (n=16) | p-value | |||
| Semen parametersb) | |||||||||||
| Volume (ml) | 3.1±1.2 | 3.7±1.5 | 0.151 | 3.4±2.8 | 3.5±2.0 | 0.682 | 3.2±0.9 | 3.1±1.5 | 0.701 | ||
| Sperm concentration (106/ml) | 42.7±18.4 | 45.7±.10.5 | 0.553 | 49.9±17.2 | 52.9±10.3 | 0.604 | 39.1±10.9 | 42.1±10.6 | 0.728 | ||
| Total motility (%) | 22.1.±7.3 | 24.1±6.2 | 0.722 | 23.6±3.5 | 23.0±5.1 | 0.916 | 20.7±8.2 | 24.0±5.1 | 0.611 | ||
| Progressive motility (%) | 12.2±3.5 | 14.9±6.1 | 0.619 | 14.0±2.8 | 14.1±4.7 | 0.738 | 10.5±5.4 | 14.8±6.0 | 0.392 | ||
| Leukocytospermia (106/ml) | 2.2±1.7 | 0.2±0.1 | 0.001 | 2.5±1.3 | 0.6±0.2 | 0.001 | 3.5±1.5 | 0.3±0.1 | <0.001 | ||
| DNA fragmentation (%) | 41.9±14.2 | 39.5±12.8 | 0.214 | 41.0±14.3 | 41.8±9.9 | 0.312 | 45.0±9.7 | 43.9±8.6 | 0.151 | ||
| Normal morphology (%) | 1.7±0.6 | 2.9±0.7 | 0.011 | 1.6±0.8 | 1.6±1.1 | 0.701 | 1.0±0.7 | 3.2±0.9 | 0.045 | ||
| Vitality (%) | 52.3±5.0 | 53.1±4.4 | 0.834 | 53.0±1.8 | 54.7±3.1 | 0.613 | 44.5±6.4 | 46.0±8.2 | 0.535 | ||
| Recurrence of bacterial infectionc) | - | 0 (0.0) | - | 1 (25.0) | - | 0 (0.0) | |||||
| P. bivia | E. faecalis | M. curtisii | |||||||||
| Pre-Tx (n=74) | Post-Tx (n=74) | p-value | Pre-Tx (n=17) | Post-Tx (n=17) | p-value | Pre-Tx (n=4) | Post-Tx (n=3) | p-value | |||
| Semen parametersb) | |||||||||||
| Volume (ml) | 2.5±1.4 | 3.0±1.2 | 0.173 | 2.1±0.8 | 2.5±1.4 | 0.383 | 3.1±0.7 | 3.5±0.5 | 0.415 | ||
| Sperm concentration (106/ml) | 31.6±19.1 | 47.9±19.1 | 0.039 | 29.8±11.3 | 51.0±13.2 | 0.022 | 44.0±12.5 | 43.1±13.0 | 0.901 | ||
| Total motility (%) | 16.4±11.7 | 49.3±12.6 | <0.001 | 23.7±4.4 | 40.8±13.4 | <0.001 | 22.4±6.1 | 25.1±7.2 | 0.730 | ||
| Progressive motility (%) | 7.8±4.7 | 39.7±11.8 | <0.001 | 13.6±3.9 | 31.3±5.1 | <0.001 | 11.1±2.7 | 16.3±4.0 | 0.526 | ||
| Leukocytospermia (106/ml) | 3.6±1.1 | 0.3±0.2 | <0.001 | 3.3±1.4 | 0.4±0.3 | <0.001 | 2.6±1.5 | 0.3±0.3 | <0.001 | ||
| DNA fragmentation (%) | 45.1±11.1 | 33.6±9.5 | <0.001 | 43.0±12.4 | 41.7±18.0 | 0.001 | 41.6±7.8 | 41.4±10.8 | 0.796 | ||
| Normal morphology (%) | 1.8±0.5 | 2.0±1.3 | 0.127 | 1.5±1.1 | 1.6±0.9 | 0.636 | 1.6±0.4 | 1.6±0.9 | 0.659 | ||
| Vitality (%) | 48.7±7.2 | 58.0±4.1 | 0.029 | 49.9±5.3 | 59.2±8.4 | 0.018 | 44.9±5.7 | 47.4±9.2 | 0.627 | ||
| Recurrence of bacterial infectionc) | - | 0 (0.0) | - | 0 (0.0) | - | 1 (25.0) | |||||
| Semen parameters | |||||||||||
| Volume (ml) | 2.1±0.9 | 3.0±1.7 | 0.140 | 2.9±1.7 | 3.1±1.4 | 0.507 | |||||
| Sperm concentration (106/ml) | 30.9±16.6 | 32.1±12.6 | 0.609 | 43.3±17.4 | 54.1±15.3 | 0.047 | |||||
| Total motility (%) | 22.1±8.6 | 41.0±10.8 | <0.001 | 20.4±10.0 | 35.8±8.2 | 0.031 | |||||
| Progressive motility (%) | 13.3±4.8 | 32.3±6.1 | <0.001 | 11.1±5.3 | 26.5±6.5 | 0.027 | |||||
| Leukocytospermia (106/ml) | 4.0±0.9 | 0.5±0.2 | <0.001 | 2.8±1.9 | 0.4±0.2 | 0.014 | |||||
| DNA fragmentation (%) | 46.4±8.1 | 42.1±10.1 | 0.129 | 43.2±13.8 | 37.6±10.4 | 0.001 | |||||
| Normal morphology (%) | 1.0±0.7 | 1.2±0.5 | 0.517 | 1.5±1.2 | 1.8±1.0 | 0.464 | |||||
| Vitality (%) | 44.4±5.9 | 46.3±11.7 | 0.805 | 51.7±6.1 | 51.1±7.8 | 0.655 | |||||
| Recurrence of bacterial infectionc) | - | 0 (0.0) | 11 (6.1) | ||||||||
Values are presented as mean±standard deviation or number (%).
U. parvum: Ureaplasma parvum, U. urealyticum: Ureaplasma urealyticum, M. genitalium: Mycoplasma genitalium, G. vaginalis: Gardnerella vaginalis, Tx: treatment, DNA: deoxyribonucleic acid, A. vaginae: Atopobium vaginae, GBS: Streptococcus agalactiae, P. bivia: Prevotella bivia, E. faecalis: Enterococcus faecalis, M. curtisii: Mobiluncus curtisii, C. trachomatis: Chlamydia trachomatis, –: not available.
a)Genitourianry tract bacterial presence indicates bacterial infection confirmed in urine or semen samples. b)Semen parameters are presented in mean±standard deviations. c)Recurrence rate was evaluated at 3 months after the initial antibiotics treatment. d)Post-antibiotics treatment semen analysis was performed on the patient who had no bacterial reinfection at 3 months after the initial antibiotics treatment. e)p-vlaues <0.05 are printed in bold characters.
Logistic analysis of bacterial species significantly influencing thespecific semen parameters of subfertile males
| Semen parameter | Bacterial species | OR | 95% CI | p-valuea) | Bacterial species | OR | 95% CI | p-valuea) | |
|---|---|---|---|---|---|---|---|---|---|
| Semen volume | None | - | - | - | None | - | - | - | |
| Sperm concentration | PB | 0.177 | 0.080-0.903 | 0.002 | PB | 0.122 | 0.019-0.757 | 0.030 | |
| EF | 0.110 | 0.045-0.691 | 0.011 | EF | 0.254 | 0.022-0.823 | 0.039 | ||
| Motility | UP | 0.119 | 0.009-0.618 | 0.021 | UP | 0.349 | 0.016-0.846 | 0.032 | |
| UU | 0.185 | 0.014-0.722 | 0.017 | UU | 0.245 | 0.119-0.531 | 0.040 | ||
| MG | 0.103 | 0.034-0.545 | 0.033 | MG | 0.170 | 0.084-0.789 | 0.041 | ||
| PB | 0.017 | 0.005-0.427 | 0.019 | PB | 0.085 | 0.011-0.332 | 0.027 | ||
| EF | 0.072 | 0.005-0.167 | 0.035 | EF | 0.590 | 0.107-1.092 | 0.077 | ||
| DNA fragmentation | UP | 0.090 | 0.023-0.125 | 0.024 | UP | 0.151 | 0.083-0.199 | 0.036 | |
| UU | 0.078 | 0.012-0.153 | 0.012 | UU | 0.178 | 0.028-0.307 | 0.025 | ||
| MG | 0.193 | 0.091-0.710 | 0.042 | MG | 0.406 | 0.091-1.185 | 0.106 | ||
| PB | 0.088 | 0.060-0.109 | 0.005 | PB | 0.091 | 0.044-0.128 | 0.017 | ||
| Normal morphology | GBS | 0.091 | 0.080-0.096 | 0.036 | GBS | 0.011 | 0.031-1.202 | 0.105 | |
| Vitality | PB | 0.122 | 0.056-0.451 | 0.013 | PB | 0.134 | 0.038-0.703 | 0.029 | |
| EF | 0.011 | 0.003-0.738 | 0.047 | EF | 0.023 | 0.010-0.512 | 0.047 |
OR: odds ratio, 95% CI: 95% confidence interval, PB: Prevotella bivia, EF: Enterococcus faecalis, UP: Ureaplasma parvum, UU: Ureaplasma urealyticum, MG: Mycoplasma genitalium, GBS: Streptococcus agalactiae.
a)p-vlaues <0.05 are printed in bold characters.
Values are presented as mean±standard deviation (range), number (%), or mean±standard deviation. LUTS: lower urinary tract symptoms, FSH: follicle-stimulating hormone, LH: luteinizing hormone, DNA: deoxyribonucleic acid, RBCs/HPF: red blood cells/high-power field, PCR: polymerase chain reaction, HSV1: Herpes simplex type-1, HSV2: Herpes simplex type-2, –: not available. a)p-vlaues <0.05 are printed in bold characters. b)Multi-microbial indicates equal or more than 2 bacterial species present in body fluid. c)1 drink is equivalent to 1 glass of wine or 1 single spirit. d)5-alpha reductase inhibitor was used for the treatment of androgenic alopecia.
Values are presented as number (%). C. trachomatis: Chlamydia trachomatis, U. parvum: Ureaplasma parvum, U. urealyticum: Ureaplasma urealyticum, M. genitalium: Mycoplasma genitalium, G. vaginalis: Gardnerella vaginalis, A. vaginae: Atopobium vaginae, GBS: Streptococcus agalactiae, P. bivia: Prevotella bivia, E. faecalis: Enterococcus faecalis, M. curtisii: Mobiluncus curtisii, N/A: not applicable, –: not available.
Values are presented as mean±standard deviation or number (%). U. parvum: Ureaplasma parvum, U. urealyticum: Ureaplasma urealyticum, M. genitalium: Mycoplasma genitalium, G. vaginalis: Gardnerella vaginalis, Tx: treatment, DNA: deoxyribonucleic acid, A. vaginae: Atopobium vaginae, GBS: Streptococcus agalactiae, P. bivia: Prevotella bivia, E. faecalis: Enterococcus faecalis, M. curtisii: Mobiluncus curtisii, C. trachomatis: Chlamydia trachomatis, –: not available. a)Genitourianry tract bacterial presence indicates bacterial infection confirmed in urine or semen samples. b)Semen parameters are presented in mean±standard deviations. c)Recurrence rate was evaluated at 3 months after the initial antibiotics treatment. d)Post-antibiotics treatment semen analysis was performed on the patient who had no bacterial reinfection at 3 months after the initial antibiotics treatment. e)p-vlaues <0.05 are printed in bold characters.
OR: odds ratio, 95% CI: 95% confidence interval, PB: Prevotella bivia, EF: Enterococcus faecalis, UP: Ureaplasma parvum, UU: Ureaplasma urealyticum, MG: Mycoplasma genitalium, GBS: Streptococcus agalactiae. a)p-vlaues <0.05 are printed in bold characters.

 KAUTII
KAUTII
 ePub Link
ePub Link Cite
Cite