Articles
- Page Path
- HOME > Urogenit Tract Infect > Volume 17(2); 2022 > Article
- Review Comprehensive Review of COVID-19 on Benign Prostate Hyperplasia Patient Symptoms
-
Joongwon Choi
 , Hong Jin Suh
, Hong Jin Suh , Dong Hwan Lee
, Dong Hwan Lee , Tae-Kon Hwang
, Tae-Kon Hwang , Jung Jun Kim,
, Jung Jun Kim,
-
Urogenital Tract Infection 2022;17(2):31-35.
DOI: https://doi.org/10.14777/uti.2022.17.2.31
Published online: August 31, 2022
Department of Urology, The Catholic University of Korea, Incheon St. Maryʼs Hospital, Incheon, Korea
-
Correspondence to: Jung Jun Kim,
 , Department of Urology, The Catholic University of Korea, Incheon St. Maryʼs Hospital, 56 Dongsu-ro, Bupyeong-gu, Incheon 21431, Korea, Tel: +82-32-280-5850, Fax: +82-32-280-5556, E-mail: metalik@hanmail.net
, Department of Urology, The Catholic University of Korea, Incheon St. Maryʼs Hospital, 56 Dongsu-ro, Bupyeong-gu, Incheon 21431, Korea, Tel: +82-32-280-5850, Fax: +82-32-280-5556, E-mail: metalik@hanmail.net
Copyright © 2022, Korean Association of Urogenital Tract Infection and Inflammation. All rights reserved.
This is an open-access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 486 Views
- 2 Download
Abstract
- Since the outbreak of the global Coronavirus disease (COVID-19) pandemic in 2019, the number of confirmed cases has increased steadily worldwide. The most common symptom of COVID-19 (SARS-CoV-2) is respiratory symptoms. On the other hand, increased voiding frequency and lower urinary tract symptoms (LUTS) have also been reported. Regarding the relationship between LUTS and COVID-19, only small size (n<100) retrospective studies have been reported, but the post-International Prostate Symptom Score (IPSS) increases compared to pre-IPSS after a COVID-19 infection in those older than 50 years. α-blockers and phosphodiesterase-5 inhibitors are relatively safe, but there are conflicting reports on 5α-reductase inhibitors; hence, further research is needed. Four major theories have been argued regarding the relationship between LUTS and COVID-19: renin-angiotensin system-related, androgen-related, inflammation-related, and metabolic derangement-related. In conclusion, elderly male patients often have benign prostate hyperplasia as a co-morbidity, and the severity of COVID-19 is high in this group. Therefore, voiding symptoms in these patient groups is of particular concern.
INTRODUCTION
MAIN BODY
1) α-blockers
2) 5-ARIs
3) PDE-5 inhibitors
1) Renin–angiotensin system (RAS) dysregulation
2) Androgen related
3) Inflammation related
4) Metabolic derangement related
CONCLUSIONS
SUPPLEMENTARY MATERIALS
-
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
AUTHORS CONTRIBUTIONS
J.C. participated in data collection and wrote the manuscript. H.J.S., D.H.L., and T.K.H. participated in the study design and performed the statistical analysis. J.J.K. participated in the study design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
NOTES

- 1. Haghpanah A, Masjedi F, Salehipour M, Hosseinpour A, Roozbeh J, Dehghani A. Is COVID-19 a risk factor for progression of benign prostatic hyperplasia and exacerbation of its related symptoms?: a systematic review. Prostate Cancer Prostatic Dis 2022;25:27-38. ArticlePubMedPDF
- 2. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061-9; Erratum in: JAMA 2021;325:1113. ArticlePubMedPMC
- 3. Gu J, Han B, Wang J. COVID-19: gastrointestinal manife-stations and potential fecal-oral transmission. Gastroentero-logy 2020;158:1518-9. Article
- 4. Mumm JN, Osterman A, Ruzicka M, Stihl C, Vilsmaier T, Munker D, et al. Urinary frequency as a possibly overlooked symptom in COVID-19 patients: does SARS-CoV-2 cause viral cystitis? Eur Urol 2020;78:624-8. ArticlePubMedPMC
- 5. Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271-80.e8. ArticlePubMedPMC
- 6. Liang X. Is COVID-19 more severe in older men? Postgrad Med J 2020;96:426. ArticlePubMedPDF
- 7. Foo KT. Pathophysiology of clinical benign prostatic hyper-plasia. Asian J Urol 2017;4:152-7. ArticlePubMedPMC
- 8. Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci 2020;12:8. ArticlePubMedPMCPDF
- 9. Elaimeri A, Alemairy AA. Effect of COVID 19 on lower urinary track symptoms in patients with benign prostatic hyperplasia. Res Sq 2021;DOI: 10.21203/rs.3.rs-514550/v1. Article
- 10. Nabeeh H, Ibrahim A, Taha DE, Talaat M, Abdelbaky TM. Impact of COVID-19 pandemic on lower urinary tract symptoms in patients with benign prostatic hyperplasia and predictors of urine retention in such patients. Low Urin Tract Symptoms 2022;14:41-6. PubMed
- 11. Can O, Erkoc M, Ozer M, Karakanli MU, Otunctemur A. The effect of COVID-19 on lower urinary tract symptoms in elderly men. Int J Clin Pract 2021;75:e14110. ArticlePubMedPDF
- 12. Vosu J, Britton P, Howard-Jones A, Isaacs D, Kesson A, Khatami A, et al. Is the risk of ibuprofen or other non-steroidal anti-inflammatory drugs increased in COVID-19? J Paediatr Child Health 2020;56:1645-6. PubMed
- 13. Konig MF, Powell M, Staedtke V, Bai RY, Thomas DL, Fischer N, et al. Preventing cytokine storm syndrome in COVID-19 using α-1 adrenergic receptor antagonists. J Clin Invest 2020;130:3345-7. ArticlePubMedPMC
- 14. Wambier CG, Goren A, Vano-Galvan S, Ramos PM, Ossimetha A, Nau G, et al. Androgen sensitivity gateway to COVID-19 disease severity. Drug Dev Res 2020;81:771-6. PubMedPMC
- 15. Dhindsa S, Zhang N, McPhaul MJ, Wu Z, Ghoshal AK, Erlich EC, et al. Association of circulating sex hormones with inflamma-tion and disease severity in patients with COVID-19. JAMA Netw Open 2021;4:e2111398. ArticlePubMedPMC
- 16. Al-Kuraishy HM, Al-Gareeb AI, Al-Niemi MS, Al-Buhadily AK, Al-Harchan NA, Lugnier C. COVID-19 and phosphodiesterase enzyme type 5 inhibitors. J Microsc Ultrastruct 2020;8:141-5. ArticlePubMedPMC
- 17. Giorgi M, Cardarelli S, Ragusa F, Saliola M, Biagioni S, Poiana G, et al. Phosphodiesterase inhibitors: could they be beneficial for the treatment of COVID-19? Int J Mol Sci 2020;21:5338. ArticlePubMedPMC
- 18. Mondaini N. Phosphodiesterase type 5 inhibitors and COVID-19: are they useful in disease management? World J Mens Health 2020;38:254-5. ArticlePubMedPMCPDF
- 19. Chinello P, Cicalini S, Pichini S, Pacifici R, Tempestilli M, Petrosillo N. Sildenafil plasma concentrations in two HIV pati-ents with pulmonary hypertension treated with ritonavir-boo-sted protease inhibitors. Curr HIV Res 2012;10:162-4. ArticlePubMed
- 20. Dinh DT, Frauman AG, Somers GR, Ohishi M, Zhou J, Casley DJ, et al. Evidence for activation of the renin-angiotensin system in the human prostate: increased angiotensin II and reduced AT(1) receptor expression in benign prostatic hyperplasia. J Pathol 2002;196:213-9. ArticlePubMed
- 21. Dinh DT, Frauman AG, Sourial M, Casley DJ, Johnston CI, Fabiani ME. Identification, distribution, and expression of angi-otensin II receptors in the normal human prostate and benign prostatic hyperplasia. Endocrinology 2001;142:1349-56. ArticlePubMed
- 22. Izumi K, Mizokami A, Lin WJ, Lai KP, Chang C. Androgen receptor roles in the development of benign prostate hyperplasia. Am J Pathol 2013;182:1942-9. ArticlePubMedPMC
- 23. Wen S, Chang HC, Tian J, Shang Z, Niu Y, Chang C. Stromal androgen receptor roles in the development of normal prostate, benign prostate hyperplasia, and prostate cancer. Am J Pathol 2015;185:293-301. ArticlePubMedPMC
- 24. Madersbacher S, Sampson N, Culig Z. Pathophysiology of benign prostatic hyperplasia and benign prostatic enlarge-ment: a mini-review. Gerontology 2019;65:458-64. ArticlePubMedPDF
- 25. Mahmudpour M, Roozbeh J, Keshavarz M, Farrokhi S, Nabipour I. COVID-19 cytokine storm: the anger of inflamma-tion. Cytokine 2020;133:155151. ArticlePubMedPMC
- 26. Gottesman BL, Yu J, Tanaka C, Longhurst CA, Kim JJ. Incidence of new-onset type 1 diabetes among US children during the COVID-19 global pandemic. JAMA Pediatr 2022;176:414-5. ArticlePubMedPMC
- 27. Metwally AA, Mehta P, Johnson BS, Nagarjuna A, Snyder MP. COVID-19-induced new-onset diabetes: trends and techno-logies. Diabetes 2021;70:2733-44. ArticlePubMedPMCPDF
- 28. Breyer BN, Sarma AV. Hyperglycemia and insulin resistance and the risk of BPH/LUTS: an update of recent literature. Curr Urol Rep 2014;15:462. ArticlePubMedPMCPDF
REFERENCES
Figure & Data
REFERENCES
Citations

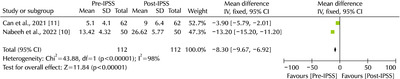
Fig. 1
Correlation between urological drugs with COVID-19
| Medication | Considerations | Drug-drug interaction related to COVID-19 |
|---|---|---|
| α-blockers | Potential to decrease acute respiratory distress syndrome. | None |
| 5α-reductase inhibitors | Mixed reports that they will play a protective or worsening role. | None |
| PDE-5 inhibitors | Theoretically, respiratory symptoms of COVID-19 can be reduced. | Avoid concomitant use with lopinavir/ritonavir. |
PDE-5: phosphodiesterase-5.
PDE-5: phosphodiesterase-5.

 KAUTII
KAUTII
 ePub Link
ePub Link Cite
Cite